Trendaavat aiheet
#
Bonk Eco continues to show strength amid $USELESS rally
#
Pump.fun to raise $1B token sale, traders speculating on airdrop
#
Boop.Fun leading the way with a new launchpad on Solana.
"What once took months now takes hours". GPT5 and other LLMs are accelerating scientific research.

17.8. klo 08.12
I’m excited to share the first part of an absolutely stunning analysis from the GPT-5 thinking model! I uploaded a huge spreadsheet, nearly 1,300 metabolites (lipids, carbohydrates, microbiome-derived compounds, and much more) measured in 150 ME/CFS patients and 100 healthy controls.
In the first run, I didn’t even tell GPT-5 these samples were from ME/CFS patients, I wanted to see what it could find blind, purely from the metabolomics data. Next, I’ll share the version where I revealed these were from our patient cohort, tied to our recently published paper and what GPT-5 uncovered there is yet on another level!
We had analyzed this same dataset over two years ago, and it took us more than a month to fully work through it.
✅GPT-5 did a better job in under five minutes.
✅It not only replicated almost everything we had concluded back then, including finding all the significant differences, creating multiple spreadsheets on different pathways and so on, but also uncovered several discoveries we completely missed.
✅GPT-5 even highlighted actionable targets and potential treatments for patients (which I’ll share soon).
This isn’t an “incremental improvement.” This is a revolution!
What once took months now takes hours. As I mentioned before the rules of scientific research aren’t just shifting, they’re being rewritten!
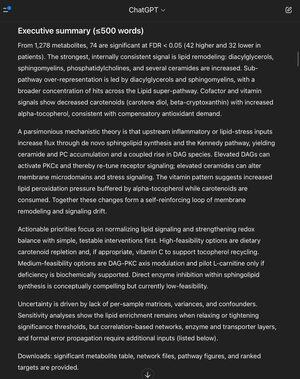
Sharing a portion of output from GPT-5 as an example, and executive summary is also included as a screenshot.
Unified mechanistic theory with causal diagram
Observed pattern
•Lipid remodeling with increased DAG, PC, SM, and specific ceramides in patients.
•Cofactor pattern with decreased carotenoids and increased alpha-tocopherol.
Mechanistic links
•De novo ceramide synthesis via serine palmitoyltransferase and ceramide synthases increases ceramide pools that influence stress and signaling.
•The Kennedy (CDP-choline) pathway couples DAG and PC metabolism; CHKA → PCYT1A → CHPT1 convert choline to PC using DAG as the acceptor.
•DAG activates PKCε and related isoforms, which can shift receptor signaling fidelity.
•Alpha-tocopherol is a lipid-phase peroxyl radical scavenger and is regenerated by ascorbate; reduced carotenoids are consistent with antioxidant consumption.
Ranked, actionable targets
1.SPTLC1/2 or CERS (enzymes) - decrease de novo ceramide synthesis. Low feasibility at present but highly causal if lipid drivers are primary. Risks include effects on myelin.
2.DGAT1/2 modulation - reduce toxic DAG signaling by shunting to neutral storage or titrating flux. Medium feasibility, GI tolerability is the key risk.
3.PKCε inhibition - block DAG-to-signaling step. Currently low feasibility, but mechanistically precise.
4.Dietary carotenoids and vitamin C support - replete antioxidant capacity and aid tocopherol recycling. High feasibility, monitor F2-isoprostanes and carotenoid panel.
5.Trial L-carnitine only if deficiency is confirmed - small signal in carnitine pathway; low-confidence, pilot dosing with monitoring.
Proposed validation experiments and minimal clinical biomarker panel
Validation experiments
•Targeted lipidomics focusing on DAG species, ceramides (chain-length resolved), sphingomyelins, PCs.
•PKCε activity proxies in accessible cells if feasible.
•Antioxidant panel: alpha-tocopherol, carotenoids, vitamin C, plus F2-isoprostanes for lipid peroxidation readout.
•If pilot L-carnitine is considered, measure free and acyl-carnitines and the acyl/free ratio pre-post.
Minimal monitoring panel
•Ceramides: d18:1/16:0, d18:1/18:0, and dihydroceramides.
•DAG class panel with positional isomers if available; report as molar % of total lipids.
•PC class and LPC/PC ratio; choline and phosphocholine to infer Kennedy pathway flux.
•Alpha-tocopherol, beta-cryptoxanthin, carotene diols, vitamin C, and F2-isoprostanes.
38,9K
Johtavat
Rankkaus
Suosikit













